Our research
Overview
Inflammation plays an important role in body defense against pathogen invasion and tissue injury, and is closely linked to serious diseases. For example, neuroinflammation is reportedly involved in nearly all neurodegenerative diseases. Our group focuses on comprehending the molecular modulation of inflammation-associated signaling pathways particularly mediated by transmembrane proteins. We aim to trace the dynamic conformational change of signal transducers by exploiting the biophysical approaches, and untangle its relationship with the function of protein machinery in combination with complementary techniques provided by biochemistry and neuroscience. Subsequently, a major goal of ours is to determine the mechanisms of the signal propagation by vital receptors across the cell membrane and its involvement in neuroimmune crosstalk and neuroinflammation, and make contributions to structure-based drug design for specific targets.
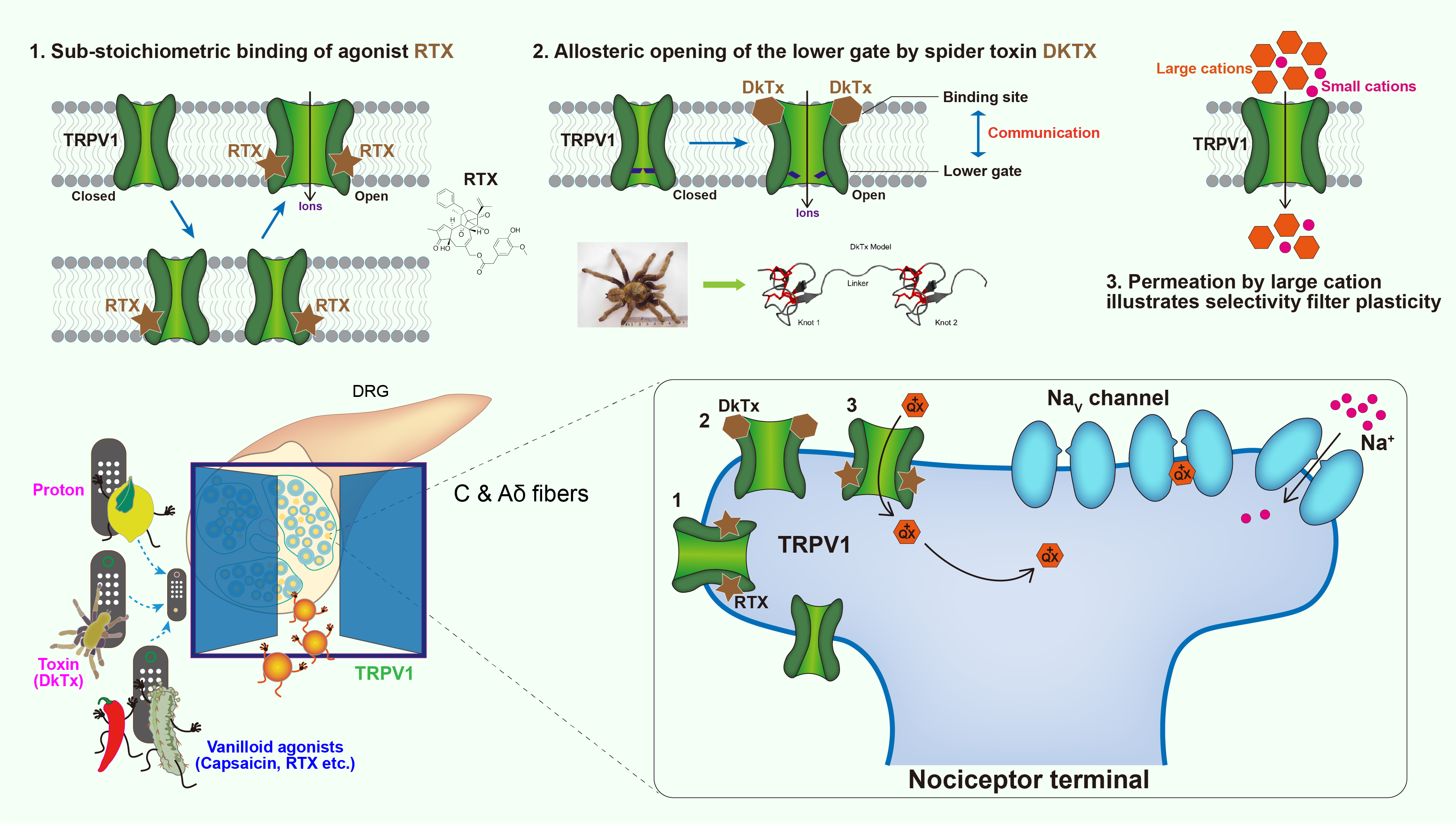
The polymodal functionality of TRPV1 revealed by structural snapshots
TRPV1 is a tetrameric ion channel primarily expressed in dorsal root ganglion (DRG) neurons, which functions in pain perception and neurogenic inflammation. TRPV1 can be activated by a range of noxious stimuli including natural products like Capsaicin actively present in chili peppers, DkTx produced by Chinese bird spiders and Resiniferatoxin (RTX) found in resin spurge. TRPV1 is also responsible for body temperature regulation through thermogenic nociception (>43 °C). We captured a series of intermediate states of TRPV1 transitioning from closed to opening when DkTx acting from pre-bound, singly-bound to double-bound, and reveal the mechanism whereby how modulation of the upper restriction by DkTx is coupled to lower gate opening. Unlike DkTx, the agonist RTX is bound in the vanilloid pocket proximal to lower gate, thereby directly inducing gating of lower restriction. we observed all possible tetrameric intermediates when vanilloids displace the endogenous lipids PI, including a single RTX, two in ortho and para positions, three and four RTX binding. Complete engagement of each RTX accordingly causes the conformational change of the ligand binding subunit. However, TRPV1 remains inactive until sufficient conformational changes take place due to fully engaged RTX in the tetrameric pockets. These findings will enhance our understanding of the function of TRP family and even other signal transducers, and provide new insights into drug development targeting TRPV1.
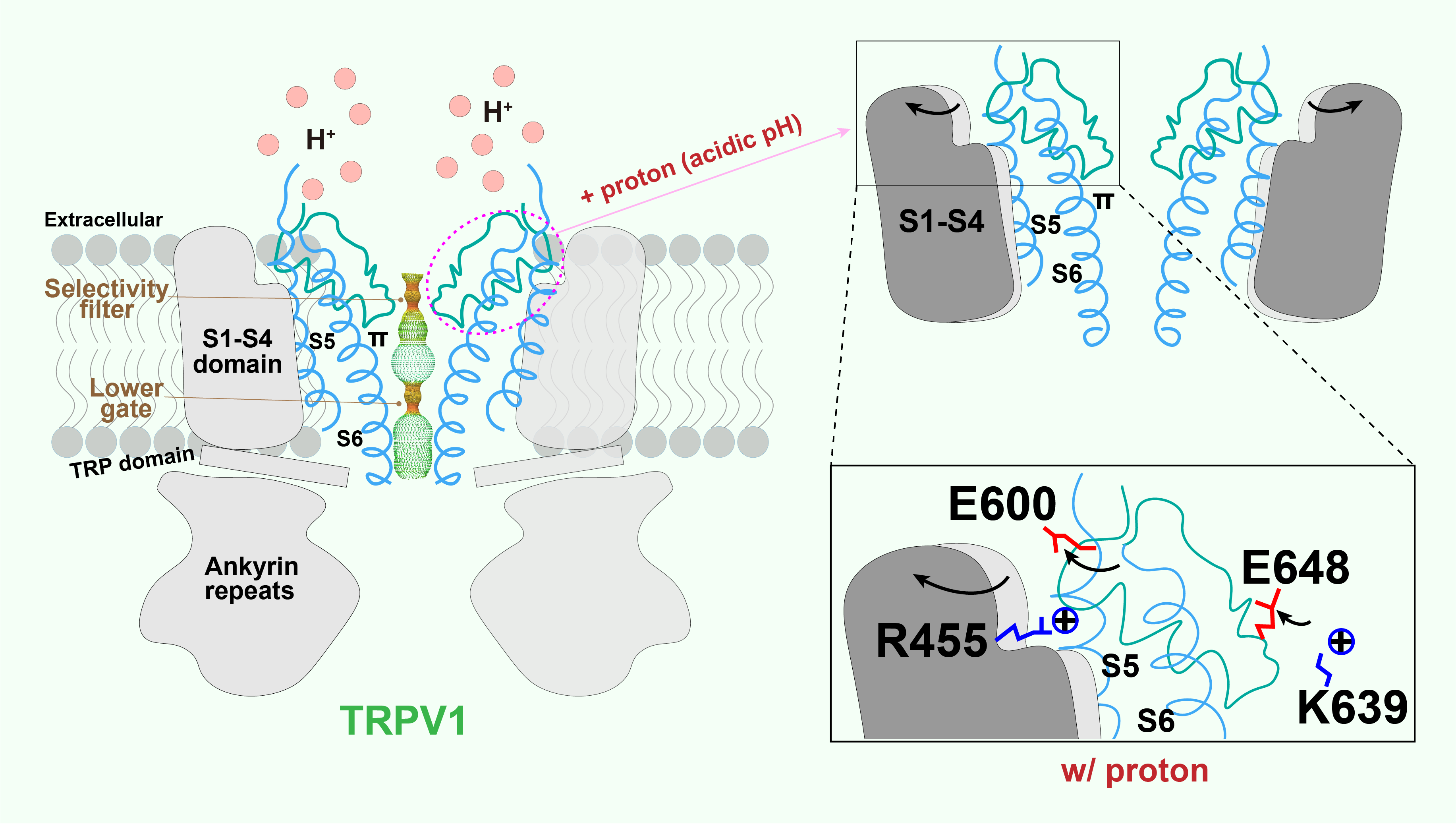
Structural mechanisms of proton action on TRPV1
Local tissue acidosis is a driver of pain hypersensitivity, which is mediated, in part, through TRPV1. Structure-function studies have identified two negatively charged residues (E600 and E648) as key sites underlying sensitivity of TRPV1 to extracellular protons. By capturing structures over a range of pH conditions, we now map conformational transitions that reveal how sequential protonation of key Glutamic acid residues facilitates movement of elements within the outer pore region and voltage sensor-like domain that promote the channel’s open state.
The agonist and antagonist recognition and function on P2Y12R
Purinergic receptor P2Y12 plays an important role in platelet activation and thrombotic disorders, which is the key target of antithrombotic drugs such as plavix, a popular inhibitor of platelet aggregation on the market. Nevertheless these drugs have side effects including indigestion and excessive bleeding etc. P2Y12R pathway is not only responsible for platelet activation, but also involved in inflammatory response and functional regulation of glial cells which has been increasingly recognized by experimental evidences. To better understand the molecular mechanisms of P2Y12R ligand recognition and facilitate drug development targeting P2Y12R, we determined the high-resolution three-dimensional structures of P2Y12R bound to antagonist AZD1283, full agonist 2MeSADP and partial agonist 2MeSATP, respectively. These results, combined with computer docking experiments, suggest that residue C97, not forming a disulfide bridge, belongs to a covalent binding pocket for the active metabolites of P2Y12R drugs. These observations are consistent with the absolute selectivity of the active metabolites. The hypothetical binding modes of P2Y12R enhance our understanding of the ligand recognition of purinergic receptors and the function of GPCR superfamily. Although both 2MeSADP and AZD1283 bind to the same pocket, their orientations are completely different, with only partial overlap between them, in contrast with the basically overlapping orientations previously observed for other receptors. Surprisingly, as a first example of a GPCR, there are striking conformational changes between nucleotide and nonnucleotide ligand complexes in the extracellular regions, as the agonist-bound pocket is sealed by a ‘lid’ formed by unusually cationic ECLs and the N terminus. These findings provide useful insights into GPCR activation and the diversity of the GPCR-mediated cell signaling.